Sustainability the key to engaging millennials
In an ironic twist Millennials, known for their high-level consumerism, are leading consumer demand for sustainably and ethically resourced raw materials in an effort to halt further environmental degradation. Sometimes referred to as the “Snowflake Generation” for having lower levels of psychological resilience than previous generations, the Millennials are using their consumer power to lead brand and consumer change.
For TransChem, sustainability means more than stopping climate change or further diminishing the world’s natural resources; it’s also about the social and economic impact and longitudinal sustainability of raw materials. Think saving the environment one ingredient at a time.
 Sustainability refers to far more than long term provision of an ingredient; it encompasses the social and ethical impact of sourcing, as well as associated causal environmental effects. The methods used to grow and produce nutraceutical ingredients can have a major environmental, social and economic impact on the local communities. The strict selection criteria applied during TransChem’s ingredient certification and validation process continually evolves to include sustainable farming methods and ethical collaborating with local communities and workers. Sustainable systems promote practices and methods that are not only economically profitable but that benefit local communities as well. Innovative and collaborative methods of sustainably growing, harvesting or producing raw ingredients ensure sufficient resources for the current generation without compromising the ability of Millenials and future generations to meet their demands. The provenance of raw materials is a TransChem core value. Adherence to the TGA’s tough requirements is mandatory but TransChem’s own investigations into the origin of new raw materials reflects the additional level of corporate citizenship around socially and ethically sourcing that consumers are demanding.
Sustainability refers to far more than long term provision of an ingredient; it encompasses the social and ethical impact of sourcing, as well as associated causal environmental effects. The methods used to grow and produce nutraceutical ingredients can have a major environmental, social and economic impact on the local communities. The strict selection criteria applied during TransChem’s ingredient certification and validation process continually evolves to include sustainable farming methods and ethical collaborating with local communities and workers. Sustainable systems promote practices and methods that are not only economically profitable but that benefit local communities as well. Innovative and collaborative methods of sustainably growing, harvesting or producing raw ingredients ensure sufficient resources for the current generation without compromising the ability of Millenials and future generations to meet their demands. The provenance of raw materials is a TransChem core value. Adherence to the TGA’s tough requirements is mandatory but TransChem’s own investigations into the origin of new raw materials reflects the additional level of corporate citizenship around socially and ethically sourcing that consumers are demanding.
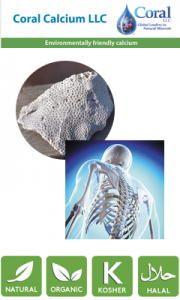 The forensic level of these investigations throws up some real gems. The harvesting of above sea coral by Coral LLC Calcium aims to maintain the pristine waters and surrounding environment of the Caribbean. Coral Calcium has been recently added to TransChem’s new ingredient portfolio. From the seabed thousands of years ago, Above Sea Coral was pushed above the tideline by the movement of tectonic plates and nature in a period before industrialisation and ocean pollution. Coral LLC sources Coral Calcium from fossilised coral on one of numerous islands with large coral deposits. Estimates suggest that by the time these current deposits are depleted, more islands will have been pushed skyward by tectonic movement, thereby supporting the long-term ecological balance of the resource. Coral Calcium is certified “Friend of the Sea” and “EcoSafe” because Coral LLC’s harvesting process does not harm the surrounding ocean’s delicate ecosystem.
The forensic level of these investigations throws up some real gems. The harvesting of above sea coral by Coral LLC Calcium aims to maintain the pristine waters and surrounding environment of the Caribbean. Coral Calcium has been recently added to TransChem’s new ingredient portfolio. From the seabed thousands of years ago, Above Sea Coral was pushed above the tideline by the movement of tectonic plates and nature in a period before industrialisation and ocean pollution. Coral LLC sources Coral Calcium from fossilised coral on one of numerous islands with large coral deposits. Estimates suggest that by the time these current deposits are depleted, more islands will have been pushed skyward by tectonic movement, thereby supporting the long-term ecological balance of the resource. Coral Calcium is certified “Friend of the Sea” and “EcoSafe” because Coral LLC’s harvesting process does not harm the surrounding ocean’s delicate ecosystem.
Verifying the origin and retaining an unbroken chain of authenticity throughout the ingredient supply process is a long established TransChem practice. Sustainably farmed ingredients, sourced from trusted suppliers who have endured rigorous levels of investigation is matched by corporate awareness of ethical practices for workers and the transparency of the entire sourcing process.
Identifying and tracking materials with detailed levels of documentation fulfil the rigorous requirements of the TGA, but for TransChem it’s about more than rules and guidelines; the provenance of ingredients align its corporate values with an informed, critical thinking and acutely aware consumer market and fits the social, ethical and sustainable requirements TransChem chooses to champion.
The influence of social media on health trends
What influences product development trends and how reliable is the evidence?
TransChem meticulously reports information on a range of globally sourced, innovative and ethically derived products to its sponsors and brands, and uses strategic social media channels to reach them.

By posting timely, relevant information TransChem provides the groundwork for the development of innovative products and presentation of upcoming trends. They deliver information to brands to support their labels and their successful product marketing campaigns. TransChem’s point of difference is the accuracy of the evidence based material and information provided to its customers, sourced from a global network of trusted suppliers and following the careful assessment of information and trends presented at international industry conferences.
But how easily is the general population influenced?
For better or worse, the dominance of social media platform “influencers” to promote everything from food to holiday destinations has helped make Australia one of the globe’s largest and most active social media consumer markets. Consequently, social media undoubtedly presents massive promotional and educational opportunities for health and healthcare. Online influence sells, but brand trust and longevity boils down to the accuracy of the information presented. TransChem empowers brands, and consumers, by providing brands with evidence-based information from trusted sources to build multichannel, brand driven marketing campaigns.
In this hotly competitive marketing industry, information is gold. Building a trusted brand requires a solid foundation of science-based, accurate information. TransChem only works with ingredients supported by evidence. They rely on a meticulous, evidence-based approach to verify the investigation of ingredients and raw materials. TransChem acknowledges the intelligence of its consumers and their curiosity to access trusted information and resources.
Let TransChem help you build your brand strength using compelling products to produce optimal consumer outcomes. Use the power of science and evidence to leverage your brand and build solid brand recognition. Ingredients sourced by reputable brand suppliers, such as TransChem, will be the only marketing influence your brand requires.
… in a galaxy (not so) far, far away …
TransChem will attend SupplySide West in Las Vegas, 6 – 10 November, 2018, the largest in a series of annual global forums which significantly influence end consumer purchasing patterns in the world’s largest marketplaces.
For approximately seven weeks leading up to the event and a further seven weeks afterwards, TransChem’s Galaxy Team is exclusively dedicated to identifying, evaluating, clarifying and determining the suitability of products for its clients from a marketing, price point?/ or economical? and technical viability perspective. The higher the attrition rate, the higher the product quality available to TransChem customers.

The Galaxy Team, named to reflect the company’s mission to go above and beyond sponsor expectations in sourcing exceptional ingredients, represents a significant investment of time, resources and passion. The process of vetting prospective ingredients is a diligent and proven system of checks, filters and investigations, ensuring TransChem’s clients are offered credible, authenticated products with substantiated claims.
While the details of this checking and cross referencing process are closely guarded, it is fundamentally a deep dive into the background of potential suppliers. Many potential ingredients don’t survive the investigation, but it takes a process this refined in the hands of a skilled, academically trained team to uncover and polish the gems that are, sometimes, revealed as diamonds for the functional foods and complementary medicines markets. The Galaxy Team’s modus operandi is to utilise a proven formula to uncover important new ingredients, and sometimes the magic.
Is it still viable for a distributor to invest such significant time, energy and effort into an expo like SupplySide West 2018? For TransChem it’s a simple equation: the greater their investment in comprehensive investigation, the more reliable and authoritative the products they offer their client base.
The Galaxy Team looks for functional food and nutraceutical ingredients with a point of difference such as purity, limited environmental impact, sustainability and a high eco-friendly rating. They look into the factory conditions, investigate the manufacturing company, scrutinise the quality of documentation, check and double check for alignment with the stringent requirements of the TGA, such as heavy metals and purity. The team checks for evidence based material surrounding the ingredients to support therapeutic claims and effectiveness.
Many potential candidates fall over, but that’s great news for TransChem’s customers. The more detailed the audit, the greater confidence TransChem customers have that the ingredients TransChem offers not only meet standards requirements, but are marketing standouts with the science to back their claims. In short, they’re exemplary but ultimately it’s how well they fit with TransChem’s vision and how well they fit with TransChem’s customers.
The exhaustive Galaxy Team process is conducted in the context of trending marketplace indicators across North America, Asia and Europe. Constantly communicating with customers, TransChem is aware of trending market indicators, including sustainability, continually scanning the globe for new and unique ingredients. Product label backstories and trends including lifelong health and wellness in areas of cognition, gut health, optical health and women’s health continue to inform the diversity of products on show at global expos such as SupplySide West, 2018.
FSANZ and the increasing opportunity to make functional food claims
Maximising the marketing potential of an ingredient that covers both the functional foods and complementary medicines spaces can be challenging, requiring experience and a detailed knowledge of Food Standards Australia and New Zealand (FSANZ).
With a broader reading of FSANZ codes, an orange suddenly becomes more than a fruit bowl staple; in a general sense, an orange also supports the human immune system, aids iron absorption, supports healthy blood vessel function, reduces tiredness and fatigue, and is a great source of Vitamin C.
Most countries have a regulatory body governing food standards and regulations. Countries with no legislated food standards use Codex by default. Australia’s food standards are defined by FSANZ, an independent statutory agency for which legislation is determined by the federal government and compliance is monitored by individual state and territory authorities. New Zealand’s food standards code is monitored by the Ministry for Primary Industry and its public health units.
As a legislative body FSANZ has, necessarily, prescriptive standards and codes relating to health and nutrition claims. While such rigid standards can seem daunting to brands wanting to market new products, FSANZ’s prescriptive standards relating to health claims also enable brands to educate the public about functional food products as well as promote them.

Food businesses and brands that choose to market a final food product can, in a general sense, make health claims for those products provided they are able to base their claims on one of more than 200 pre-approved health relationships specified in the FSANZ standards, or they can self- substantiate a food relationship in accordance with the FSANZ standards.
Sounds daunting? It can be.
But did you know that health claims can be made for a functional food as well as a Therapeutic Goods Administration (TGA) approved complimentary medicine? And, ironically, it’s the stringency of the FSANZ codes that’s the enabler.
Harnessing the reality of legislative obligations of the health product claims of functional foods as well as products listed under the TGA is a TransChem speciality. TransChem’s industry and academically trained team assists clients capitalise on claims, meet FSANZ food labelling compliance obligations and successfully market innovative functional food ingredients; the information and support the TransChem team offers enables brands, as well as end consumers, make better product content choices.
TransChem continues to develop its portfolio of ingredients that can support compliance in the functional foods area. It currently has more than 20 different food and beverage products from hundreds of globally sourced ingredients. TransChem’s forensically detailed investigation of potential raw materials to add to its portfolio of innovative, ethically sourced ingredients continues at SupplySide West (6-10 November, 2018) in Las Vegas.
TransChem’s expertise in this field means its team can guide clients through the health and marketing claims not only in the complementary medicine sphere but also in the functional foods space, meaning a product containing listed medicines in the complementary medicine sphere may also leverage and promote health claims.
Just like, in a general sense, the humble orange.
The complexity of consumer awareness about wellness
Increasing consumer awareness and knowledge about the benefits of healthy living and a long- term active lifestyle translates into a strong demand for new products across the dietary supplement, complementary medicine, pharmaceutical, functional food and FMCG product ranges. Consumer trends have also revealed that consumers are more mindful of sustainability and ethical sourcing when choosing products and brands.

The challenge for the listed complementary medicine industry is to not only supply this demand, but lead and inform consumers with high quality, innovative and ethically sourced raw materials and ingredients for new products to treat evolving health issues.
TransChem is well placed to meet this challenge with a significant point of difference in the industry. TransChem’s team understands these demands and frustrations and actively engages with brands to source the innovative, ethical, environmentally sustainable and claims- substantiated ingredients and raw materials required for successful new products. A recent example of this from Vitafoods Asia 2018 is TransChem’s engagement as distributor for a particularly unique Icelandic source of astaxanthin, an ingredient that is the king of antioxidants along with other therapeutic benefits, which crosses the blood-brain barrier and may benefit cognitive and optic performance.
As a recognised supplier of high quality raw materials and ingredients and with a global matrix of trusted suppliers, TransChem has a proven track record of supporting brand sponsors’ NPD. Across the nutraceutical industry, NPD requires continual upgrading of current formulations. The constant search by consumers for new and different ways to attain, or maintain, healthy ageing and vitality applies pressure to brands, manufacturers and sponsors for market readiness.
Diligent scientific verification always trumps the market spend on new products at TransChem. Their innovative new ingredients undergo a rigorous process to confirm efficacy, quality and relevance to the Australian market.
Brand sponsors are not always informed of all the ingredients available; they recognise the problems and issues consumers want solved but are not always completely aware of the new ingredients available that may address these issues.
TransChem will continue to track global nutraceutical trends and product innovation at the November 2018 SupplySide West event in Las Vegas.
It’s all in the detail
Detailed, supported product and marketing information are essential elements for brand owners and sponsors in the listed complementary medicine product development process. Qualified, evidence-based product and ingredient verification means they do not need to develop that aspect of new marketing campaigns.

Specific and detailed product or ingredient features, as well as product knowledge by the manufacturer, leads to brand reliability and trust in the marketplace, but it’s not easily attained. Evolutionary new product development and the important role played by innovative ingredients gives TransChem customers, both contracted manufacturers and brand owners, a competitive edge when it comes to detailed product knowledge.
Brand manufacturers want products that demonstrate developed and supported ingredient claims and benefits, as well as appropriate marketing support material, to market and better position their product to end consumers.
Essentially, sponsors want ingredients that sell themselves; they want ingredients with a pool of leverageable claims supported by readily available, TGA compliant evidence. Without industry experience, expertise and high level service support, this is not a simple equation.
When TransChem customers source ingredients, the products are backed by detailed marketing information and leveraged, evidence-based and supported claims. This knowledge is underscored by a deep understanding of client needs and is part of a suite of customer service operations TransChem offers to assist branded product development and effective end consumer marketing.
TransChem is well placed to provide its customers with detailed, reliable information: it has a broad, long standing international product sourcing network, together with a close and trusted stable of suppliers of innovative ingredients. It maintains constant communication with ingredient suppliers and regularly attends international conferences and trade shows researching the background of both the innovative ingredients and suppliers.
It would be unusual for a stand alone brand to have this international scope, reach or expertise and would take years to replicate. TransChem’s longstanding relationships with international suppliers provides brands with a true competitive edge, guaranteeing both brands and manufacturers ingredient innovation, verification, traceability and quality, effectively slipstreaming them towards end stage new product development.
Professionally qualified and with cumulative decades of industry experience supplying raw materials and high quality ingredients to the pharmaceutical, food, beverage, animal health, personal care, complementary medicine and dietary supplements industries, TransChem’s staff offer an international perspective and understanding of the nutraceutical industry and are well positioned to assist brands substantiate new product knowledge from a technical, marketing and regulatory perspective.
It’s going to be like finding a needle in a haystack
In one of three annual conferences where the global nutraceutical industry congregates to showcase, discover and explore quality ingredients, raw materials and innovation, more than 300 exhibitors from 60 plus countries will fill the pavilions at Vitafoods Asia, Marina Bay Sands, Singapore 11-12 September, 2018.

Travelling to the Vitafoods Asia event with a full book of appointments is TransChem CEO Andrew Klapka. These booked appointments are not just any dance card partners; with a celebrated reputation across Australia and New Zealand for the quality of their ingredients and raw materials, TransChem’s highly qualified appointments have already undergone weeks of rigorous filtering and exploration.
TransChem instigates an eight week pre qualification process for these global events with a dedicated project team of between three and four staff carrying out meticulous background research and investigation of the ingredients and raw material products on offer.
The rigour of this proven culling process reduces the hundreds of exhibitors to, perhaps, the top 50 exhibits of sufficiently high quality to pique TransChem’s interest.
A carefully defined evaluation model follows to uncover suppliers genuinely wanting to distribute in Australia and New Zealand, the differentiating appeal of the ingredients, and the ingredients and raw materials that meet strict TGA regulatory guidelines.
From this highly specific culling process there could be between just five and ten ingredients sufficiently well qualified for TransChem to followup on.
Understanding what’s on show at these global industry events and how any of the displayed products correspond with the specific needs, and sometimes unresolved needs, of their clients means TransChem will also canvas suppliers, looking for specific ingredients of interest.
From the hundreds of products on display, it takes a trained eye and a deep understanding of TransChem clients’ “wish lists” to differentiate exactly what’s on show, how it corresponds with customer requirements, and whether or not it meets TransChem’s exacting high quality standards
It takes time to be “first to market”
Over saturation and keen competition in the end consumer market for dietary supplements and complementary medicines drives constant sourcing demands and a perceived need for “first to market” claims.
With a seemingly limitless array of raw materials and nutraceutical product ingredients available globally, quality brands and manufacturers rely on meticulous filtering processes to reveal reliable, innovative and approved elements.

Why is “first to market” so important?
For manufacturers and brands, achieving “first to market” is a key factor for product success; it’s a great selling point, it demonstrates to consumers that the brand is on trend, and it enables a brand owner to claim that marketing advantage. Brand owners are constantly seeking product range extensions or new products to launch. New or improved ingredients are critical to brand growth and development, and making new claims for an ingredient provides the opportunity for product line extension.
For quality brands and manufacturers, attaining “first to market” status is not a race. By forming strategic partnerships with suppliers, such as TransChem, to identify credible, unique, high quality, innovative and traceable ingredients for their products, claiming “first to market” comes at the end of an extensive investigation process.
TransChem’s CEO Andrew Klapka will attend Vitafoods Asia in Singapore, 11-12 September 2018. With a full book of appointments with suppliers, Mr Klapka will be looking for particular ingredients of interest to TransChem clients and canvasing exhibitors for ingredients of interest, beginning an estimated one to three month process of culling and filtering which aims to verify between five and 10 well qualified ingredients from the hundreds of raw materials and ingredients on offer.
It’s a painstaking process that has earned TransChem an enviable reputation as the leading independent importer and distributor of quality raw materials across Australia and New Zealand; TransChem provides “first to market” branded ingredients to create new products and clients can be confident of the quality, differentiating appeal and innovation of their ingredients.
TransChem works with a brand, sponsors and manufacturers to identify and source new to market and market leading ingredients based on industry insight, brand and consumer feedback. Specialist supplier partnerships are helpful for brands to maintain this constant capability and exhibit a point of difference; they enable brands to focus on product development, thus supporting brands to make the “first to market claim”. It’s like fitting a Research & Development booster into the manufacturing process.
By partnering with TransChem, brands and manufacturers have the line of extension option; TransChem assists with product Research and Development and the client benefits from TransChem’s established industry experience and inside knowledge of the ingredient industry.
These symbiotic relationships assist brands or manufacturers with the time-consuming process of searching for innovative ingredients and evaluating reliability of supply and costing. By being involved in early-stage New Product Development, TransChem guarantees not only ingredient traceability and quality assurance but, crucially, ingredient relevance and reliability.
Traceability, Reliability, Security; TransChem’s Modus Operandi
Sourcing ingredients and raw materials is no longer enough. In a marketplace where manufacturers and consumers demand quality and assurance, provenance is key. The mission statement of the leading independent importer and distributor of high-quality raw ingredients into Australia and New Zealand, TransChem, focuses on the simple fundamentals of guaranteeing the secure and reliable supply of innovative, highest quality materials to give its clients the edge.
With networks worldwide and proven sourcing techniques, TransChem is supplying quality raw materials for products as diverse as pharmaceutical actives, complementary medicines, food and beverage, and stockfeed items to Australia’s manufacturing doorstep.
TransChem proactively supports its clients’ business success by developing deep, long-term client relationships with expertly sourced and reliably supplied, quality raw materials at competitive prices. TransChem also specialises in sourcing innovative functional ingredients, involving unique and patented processes, which then act as differentiators for their clients’ finished products.
Intuitive recognition of market needs stimulates the sourcing of relevant ingredients. Many items in TransChem’s ingredient range are under exclusive supply arrangement, delivering TransChem clients an immediate market advantage. Ultimately, it’s a thorough understanding of what the market needs, driven by actively listening to the details of what customers are working on in their new product development programs that drives TransChem’s international sourcing efforts. No matter how remote the location or how specific or unique the ingredient, TransChem’s global network will track it down; from active pharma ingredients in Germany, to flavours from Slovenia or yam extracts from Ecuador, with contacts spanning Europe and the USA, TransChem’s network will pinpoint them on the map.
Certainty about the chain-of-integrity and quality of ingredients from farm to finished product is an integral TransChem value and a significant point of difference in the market.
Customers can rely on TransChem to provide only the very best quality ingredients from the most reliable overseas suppliers. It’s an enviable reputation built on decades of careful and methodical attention to detail. At TransChem, it’s the little things that count.
TransChem guarantees the traceability and quality assurance of the ingredients it supplies by diligently implementing ‘vendor assurance questionnaires’ which strictly apply the Australian Self Medication Industry (ASMI) standards. These questionnaire protocols are the product of collaboration between the Complementary Medicine Association of Australia (CMA) and ASMI to ensure strict standards apply to the vendor qualification of suppliers of raw material ingredients for the manufacture of medicinal products.
While the responsibility for guaranteeing ingredient relevance and reliability rests with the client, TransChem actively assists clients by providing technical support along with the appropriate supporting documentation. This attention to detail and early stage support is a point of difference TransChem is proud of; caring, service and knowledge are all key TransChem values.
Time is of the essence in TransChem’s supplier relationships and this not only relates to the timely delivery of materials; time is invested with newly recruited suppliers during face-to-face meetings, particularly at international trade fairs. Interviews and in-depth discussions with potential new suppliers at these forums are informed by decades of experience, honed to reveal the background stories of suppliers, their view of themselves in their own words, and to weed out short-cuts and unreliability of supply.
TransChem has more than 30 global partners providing a network of contacts and advisers who have worked exclusively alongside them for more than 20 years. These are major partners and include well-known global brands from Switzerland, Italy, Japan, USA, Netherlands, Canada, Taiwan and South Korea, as well as some of the highest quality and established suppliers from both China and India. TransChem’s point of difference here is simple; such longstanding relationships are predicated on absolute trust in the quality of process and ingredient from farm to finished product. The integrity and quality of a product can only survive if its existence and performance is real and sustainable, and TransChem strives to maintain the longevity of its global partner relationships.
TransChem works exclusively with verified international experts. Numerous suppliers have approached TransChem over the years but only those with the discipline and integrity of process required get through to first base. Stringent evaluation criteria, vendor qualification processes and compliance assessments ensure only the best suppliers are selected. Technical verification checks are performed by TransChem, ensuring clients’ peace of mind that they are using not only a high quality ingredient, but that it is from a verified, reliable and trusted source.
Valuing and maintaining close client relationships is a critical element of TransChem’s business, particularly when it comes to sourcing ingredients. This collaborative approach means being well informed about new product development, studying core trends within various consumer segments, knowing the requirements of the export markets served by clients and understanding which new ingredients and their associated functionalities are working in which overseas markets.
This deep understanding of the market drives the company’s global search for increasingly innovative raw materials and to source the highest quality ingredients from the most reliable overseas suppliers.
Setria® – a new wave of interest



Setria is riding a new wave of interest in Australia and New Zealand, as a growing number of nutraceutical companies begin to utilise this unique tripeptide in promoting detoxification. Such is the interest, Kyowa, who manufacturer Setria Glutathione®, sent three executives to the Complementary Medicines Australia (CMA) 2018 Innovation Seminar and Supplier Expo, to address a keen audience.
Setria is made up of three amino acids: glutamate, cysteine, and glycine. It helps fight off free-radicals and toxins, provides antioxidant protection, promotes detoxification, fortifies the immune system and brightens the skin. It is manufactured through a patented fermentation process to GMP standards.
TransChem, are the exclusive local distributors of Setria and have been championing the product for the last three years since its initial release here, promoted as the Master Antioxidant. The CMA Expo presented an opportunity for the two companies to combine for a stand dedicated to showcasing the benefits of the product.
 According to TransChem Commercial Manager Lewis Tessarolo “we fielded a number of questions about Setria. It was wonderful to have Kyowa’s Director of Global Brand Marketing Karen Todd, Deputy Sales Manager Cicilia Amex and Senior Director Raymond Goh from Singapore on hand.”
According to TransChem Commercial Manager Lewis Tessarolo “we fielded a number of questions about Setria. It was wonderful to have Kyowa’s Director of Global Brand Marketing Karen Todd, Deputy Sales Manager Cicilia Amex and Senior Director Raymond Goh from Singapore on hand.”
Karen Todd, who is based in New York, presented at what CMA called its ‘Innovation Day’. As a Registered Dietician and Certified Strength and Conditioning Specialist and Health & Fitness Specialist with more than 25 years experience, she was able to relate the value of the ingredient on both a technical and practical level.
Setria is supported by a milestone trial which demonstrated an increase in glutathione levels in the blood through the use of oral supplements.
Lewis says, “The reason it is becoming more and more popular is the strong science and marketing tools to support Setria is exceptional so as an ingredient it maximises the potential reach”.
Setria can be used in capsules, tablets and powders or sachets.

 +61 (0) 2 9887 1688
+61 (0) 2 9887 1688


